College of Engineering Unit:
Radionuclides are an essential part of the treatment and imaging of cancers and other aggressive diseases. They are a crucial aspect of medicine, and the demand for them is rising as their potential is realized. One of the most promising new radionuclides used in medicine is Lutetium (Lu)-177, as it has become integral in diagnostic and therapeutic procedures. Many of the sources of medicinal grade Lu-177 are located outside of the United States. As a result, the supply chain is very delicate and subject to extreme swings in price and availability. Therefore, there is an ever-growing need for domestically produced radionuclides. Increasing the production of these materials within the United States would shorten the shipment routes, which will decrease the amount of Lu-177 lost to decay. Domestic production would also increase the supply of Lu-177 available for therapeutic use, making these treatments more readily available to those who need them and reducing the cost. For these reasons, it is crucial to expand the number of designated facilities producing Lu-177 as well as the number of research reactors investigating their radionuclide production capabilities. This project analyzes the feasibility of the Oregon State University TRIGA Reactor (OSTR) creating the radionuclide Lu-177 with a minimum activity of 200 mCi to be used for therapeutic treatments.
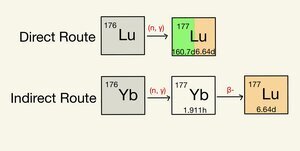
There are two production pathways to achieve Lu-177, known as the direct and indirect routes. The direct method is the irradiation of Lu-176 with neutrons to produce Lu-177. The indirect route is taking Yb-176 and irradiating it with neutrons to achieve Yb-177, which will decay to Lu-177. The direct production route's activity depends on the reactor's flux as the target and product materials are chemically similar. The indirect route activity is not dependent on the thermal flux of the reactor due to the target and the product being chemically different. This difference means very high specific activities and purity levels can be reached through post-irradiation processing. Both production routes were researched to determine the feasibility of Lu-177 production at the OSTR. Equations found in previous literature were adapted in MATLAB to provide an in-depth analysis of the production capabilities within the OSTR. Additionally, post-irradiation processing methods currently available for lanthanides were analyzed. This analysis of post-irradiation processing methods showed extraction chromatography to have particular promise and is mass producible. The separation of lanthanides is primarily based upon ion affinity and the acidity of the rinse. LN, TK, and DHA resins could be utilized within the extraction process as they are all suitable for proper separation of Lu-177 from Yb-176. The process could happen at Oregon State University if a hot cell were acquired.
These calculations showed that the indirect production route of irradiating Yb-176 in the In-Core Irradiation Tube (ICIT) is the only feasible route of Lu-177 production in the OSTR. The amount of material that can be irradiated at one time is limited to 10 grams of target material due to volume restrictions of the irradiation tubes for the ICIT facility and the need to prevent the target from self-attenuating. The irradiation time necessary to reach a minimum activity of 200 mCi has been analyzed with a range of 5-30 days. The ICIT’s maximum flux of 1.1*10^13 n/cm^2 was used to find the Lu-177 product amount when Yb-176 was irradiated for 15 days. For one gram of Yb-176, 23.71 micrograms of Lu-177 can be produced. Therefore, with the maximum limit at 10 grams of the Yb-176 target material, 0.269 milligrams of Lu-177 can be produced. This means at the OSTR's flux, 0.1752 grams of the target material are needed to produce one therapeutic dose of 200 mCi of Lu-177 after a 7-day decay period. By taking the maximum amount of mass that can be irradiated and dividing it by the mass needed for one dose, 57.08 doses can be obtained.
Project Communication Piece(s):
| Attachment | Size |
|---|---|
| 1.7 MB |