College of Engineering Unit:
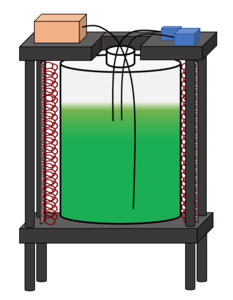
This project is a design improvement of a system currently in use in the Philmus lab at OSU. Cyanobacteria are currently being investigated for their potential to produce active pharmaceutical molecules. Cultivation is necessary to establish a relationship between bacteria genomics and their influence on the product molecules. Currently, cyanobacteria are grown in a 20 L carboy located in a temperature-controlled room. An aquarium pump bubbling air is used as a method for agitation and light is supplied with one fluorescent light on one side of the carboy. The cells are grown in BG11 media (a nutrient stock solution modeled by lake water) added at the beginning of the growth. It is hypothesized that the cyanobacteria run out of necessary nutrients throughout the process, inhibiting potential growth rate and density.
The goals of this project aim to design a photo-bioreactor that improves the growth rate and increases the cellular density of cyanobacteria during cultivation. This will be done by improving light distribution throughout the reactor and increasing nutrient availability to growing cells. The researched bacteria require specific wavelengths of light and differing amounts of light for optimal production. Four dimmable, redshifted, LED grow lights will deliver an optimal light spectrum and intensity during the various stages of growth. Constant agitation is required to prevent settling and improve light distribution. Aeration with ambient air will continue to be used as a method for agitation at an approximate rate of 6-9 L/min. Additionally, the use of ambient air in agitation will provide carbonate to the system. Nutrient concentrations will be monitored using a nitrate probe. Dissolved nitrogen concentrations will be directly read from the nitrate probe reading and phosphate concentrations will be inferred using the Redfield ratio. Nutrient availability will be accomplished via nutrient addition mid growth or by increasing the initial concentration of phosphate and nitrate nutrients in the media. A larger, 25 L, carboy will be used to accommodate media volume increases from supplemental nutrients.
To optimize system ergonomics, telescoping legs will allow height changes for ease when moving the reactor vessel for sterilization and product harvesting. To safeguard possible reactor leaks/breakage, the carboy will sit on a shelf with drainage holes, and a lower shelf will have a vessel to contain the possible spill.