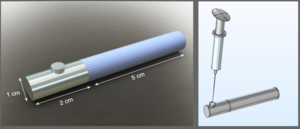
Five percent of the US population –that is, 16 million people– experience anaphylaxis per year. Anaphylaxis is a life-threatening allergic reaction that is characterized by swelling of the face, skin rashes, nausea, and difficulty breathing. Current solutions include epinephrine auto-injectors, such as Mylan's ubiquitous Epipen®, with approximately 3.6 million patient prescriptions annually. Auto-injectors administer epinephrine into the thigh of a person who is experiencing anaphylaxis to slow or block allergic response progression. However, auto-injectors are both expensive and inefficient. They must be replaced every 12-18 months, meaning patients who practice food and allergen safety often discard them prior to use. The devices are also non-reusable, and a person who is at risk for anaphylaxis must always carry one to prevent a reaction that might become fatal. We propose a solution that prevents anaphylaxis at the source – a refillable Bruton’s Tyrosine Kinase (BTK) inhibitor subcutaneous implant. BTK inhibitors are FDA approved to treat lymphoma and leukemia, but recent studies have shown that BTK inhibitors can prevent IgE-mediated anaphylaxis. Inspired by contraceptive implants, our proposed design consists of a 5 cm long, 1 cm wide cylinder rod made out of a flexible polymer such as ethylene-vinyl acetate attached to a 2 cm long refill reservoir with a silicone refill port. We developed computer models with COMSOL to determine the diffusive parameters and polymer thickness needed to reach our desired flux out of the implant, and Solidworks models were developed for strength testing of the implant. The polymer implant rod will have an outer, rate-determining polymer layer surrounding a less dense polymer layer charged with the BTK inhibitor, ibrutinib. The center is hollow and connected to the refill reservoir, which will allow for regular refills with a small syringe needle. Based on the 6% oral bioavailability of ibrutinib and currently accepted doses of ibrutinib, the implant will slowly release an average of 25 mg per day. With the proposed dimensions and parameters, the implant will release a dose in the therapeutic range for 35 days before a refill is required. Though the implant is larger than contraceptive implants on the market due to the required daily dose of ibrutinib necessary for anaphylaxis prevention, our implant leverages the benefits of preventing a life-threatening anaphylactic shock against the inconvenience of its size. Our goal is to prevent anaphylaxis from occurring and eliminate the need for an epinephrine auto-injector. Additional advantages of our implant’s form include reduced waste due to a higher drug bioavailability and blood maintenance levels within the therapeutic range.
| Attachment | Size |
|---|---|
| 497.51 KB | |
| 88.41 KB | |
| 30.27 KB |

